Creating Personalized Prescriptions for Transcranial Brain Stimulation
- Published2 Sep 2021
- Author Andrew Meissen
- Source BrainFacts/SfN
Two millennia ago, the Roman physician Scribonius Largus placed bioelectric torpedo fish on the scalps of headache sufferers until the fish’s static made them pain-free. Today, researchers are exploring transcranial direct current stimulation (tDCS), a noninvasive technique deploying battery-powered devices to transmit a painless, mild electrical current to treat a variety of conditions such as depression and schizophrenia.
The treatment is promising — one study showed tDCS increased recall of newly-learned words by 15%. But, it has one catch. Everyone has a unique head, and so a one-size-fits-all treatment protocol doesn’t benefit everyone the same way. But artificial intelligence may solve that problem.
Adam Woods, associate director of the Center for Cognitive Aging and Memory at the University of Florida, runs the largest NIH-funded study on tDCS in the United States. His laboratory uses tDCS to slow the cognitive decline that comes naturally with age. When stimulating participants’ brains, the researchers place electrodes on peoples’ foreheads to target their frontal lobes, the brain region first to degenerate with age.
Stimulation usually lasts around 20 minutes. For the sham (control) condition, participants receive 30 seconds of stimulation to mimic the sensation that something is happening — although it’s not long enough to have a significant effect on the nervous system. The participants test their skills through brain games while they receive stimulation. Many do show fewer signs of cognitive decline — especially in episodic memory and attention — than those who receive sham stimulation. Others don’t respond to the stimulation at all.
“In the realm of non-invasive brain stimulation, individual variability is a monstrous reality,” Woods says. That’s true for all treatments using electricity to penetrate the skull and rewire the brain without surgery. “You can do the exact same stimulation and this person responds, and this one doesn’t.”
That uniform methodology may underlie the problem. Woods and other scientists, including Hannah Filmer, a tDCS expert at the University of Queensland in Australia, suspect our unique brain anatomies dictate what is an effective dose of electricity and where on our scalps it is best placed. “We accept the fact there’ll be a little bit of variation as to exactly where the area we’re targeting is in that person,” Filmer says. Using the same stimulation patterns for everyone could doom the procedure to failure in some — possibly many — individuals.
“All these things are unique. They’re like a fingerprint. They’re unique person-to-person.”
“When you’re putting electrical current into the brain, there’s a whole bunch of features that relate to your head and brain that are unique to you,” Woods says. “The thickness of your skull, the amount of fatty tissue underneath the skin, the amount of cerebrospinal fluid in the brain from, let’s say an aging atrophy process versus a young healthy brain, the unique folds of gyri and sulci of the brain. All these things are unique. They’re like a fingerprint. They’re unique person-to-person.”
Filmer, Woods, and others monitor what’s happening to the electrical current aimed at the brain with magnetic resonance imaging (MRI). “We scan them, and then run them through a tDCS study,” Filmer says. “And what we do is we say ‘Well, how much was that individual affected by stimulation? Are there any markers, any indicators as to who is going to respond particularly well, and who isn’t going to respond particularly well?’”
By collecting a database of these markers and feeding them into artificial intelligence systems, Woods’s team successfully predicted who would respond to tDCS 86% of the time in a small study of older adults. By defining individual parameters that result in an effective response, researchers seek to personalize tDCS administration to increase the benefit to each and every individual.
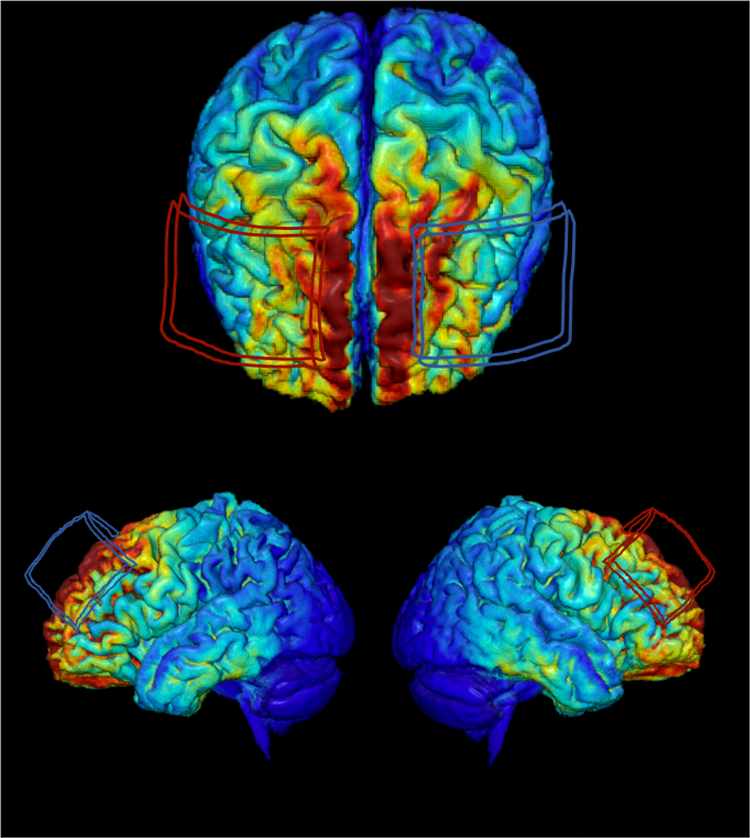
When targeting a brain region for stimulation, researchers and doctors typically match a standard circular map of a human scalp to the head of the participant. That map includes spots to place two or more electrodes. Matthew Krause, a research fellow at McGill University/Montréal Neurological Institute notes in the case of tDCS, current flows between the electrodes. The electrical current is carried through the scalp and skull and predominantly permeates the first few centimeters of the brain.
Exposing large areas of the brain to a single source of electricity makes them more likely to talk with each other. The nerve cells become receptive to signals from other nerve cells after the stimulation. The increased connectivity can induce a neuroplastic response, causing brain networks that have deteriorated with age, for example, to relearn how to work in synchronicity with healthy networks. With MRI scans of people’s brains before and after tDCS trials, researchers are using machine learning techniques to discern each person’s anatomical fingerprints and understand how a specific dose of electricity changes an individual’s brain.
With data from every research participant, Woods and his team are training algorithms to analyze the data and identify specific features which predict whether any given person will respond to stimulation. By identifying common characteristics and further identifying patterns across the responders and non-responders, Woods can make more detailed, refined templates to develop a profile of an individual, know where to put the electrodes, and determine how much current to use.
Woods and his collaborators are aiming for a “push-button” scenario where a patient walks in to treat a condition such as depression or age-related cognitive decline, receives a brain scan, and the clinician presses a button that outputs a stimulation prescription. The computer algorithm-derived prescription would dictate the exact dosage, duration, and location of stimulation specific for that person’s unique skull, brain, and symptoms. Normally, it would take Woods’ team months to give an individual such personalized attention, but if their approach is realized, with the support of neuroimaging and AI, these empowered prescriptions could be churned out the same day someone receives the MRI scan.
With an individualized approach, researchers and physicians could better understand how noninvasive brain stimulation might be used to prevent or slow cognitive decline, or positively change the course of Alzheimer’s disease and other dementias. Woods notes, “As we start to dig into this technique and really start to understand mechanistically what’s driving these differences, the better we can titrate this technique to impact more people in a positive direction.”
CONTENT PROVIDED BY
BrainFacts/SfN
References
Albizu, A., Fang, R., Indahlastari, A., O’Shea, A., Stolte, S. E., See, K. B., Boutzoukas, E. M., Kraft, J. N., Nissim, N. R., & Woods, A. J. (2020). Machine learning and individual variability in electric field characteristics predict tDCS treatment response. Brain Stimulation, 13(6), 1753–1764. https://doi.org/10.1016/j.brs.2020.10.001
Indahlastari, A., Hardcastle, C., Albizu, A., Alvarez-Alvarado, S., Boutzoukas, E. M., Evangelista, N. D., Hausman, H. K., Kraft, J., Langer, K., & Woods, A. J. (2021). A Systematic Review and Meta-Analysis of Transcranial Direct Current Stimulation to Remediate Age-Related Cognitive Decline in Healthy Older Adults. Neuropsychiatric Disease and Treatment, 17, 971–990. https://doi.org/10.2147/NDT.S259499
Meinzer, M., Jähnigen, S., Copland, D. A., Darkow, R., Grittner, U., Avirame, K., Rodriguez, A. D., Lindenberg, R., & Flöel, A. (2014). Transcranial direct current stimulation over multiple days improves learning and maintenance of a novel vocabulary. Cortex, 50, 137–147. https://doi.org/10.1016/j.cortex.2013.07.013
Pease, J. (2021, July 13). UF researchers use AI to develop precision dosing for treatment aimed at preventing dementia. UF Health. https://m.ufhealth.org/news/2021/uf-researchers-use-ai-develop-precision-dosing-treatment-aimed-preventing-dementia
Stagg, C. J., & Nitsche, M. A. (2011). Physiological Basis of Transcranial Direct Current Stimulation. The Neuroscientist, 17(1), 37–53. https://doi.org/10.1177/1073858410386614
Also In Therapies
Trending
Popular articles on BrainFacts.org