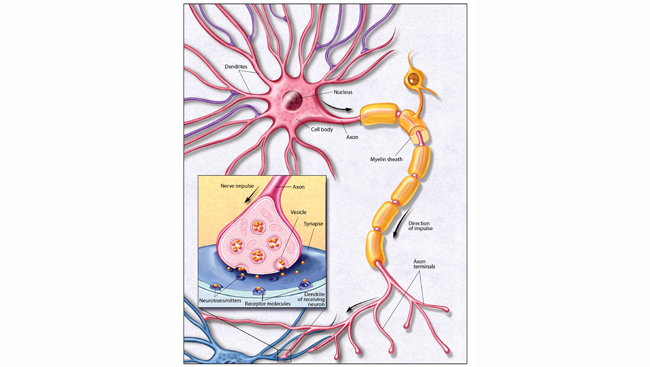
Zika and the Brain
- Published1 Mar 2016
- Reviewed1 Mar 2016
- Source The Dana Foundation
How viruses like Zika disturb fetal brain development is a long-standing mystery.
Judging by the headlines, Zika is the new Ebola — a virus that is spreading rapidly in warmer countries, and spreading fear worldwide. Of course in reality Zika is much less virulent than Ebola. While transmissible by certain mosquito species, and apparently also by sexual contact, it normally doesn’t cause much harm to adults it infects. In fact, the vast majority of infections are so benign that they aren’t even noticed. Even the infections that are symptomatic tend to be relatively mild: fever, rash, joint and muscle pain, and conjunctivitis, which usually abate after a few days.
However, Zika is thought in some cases to cause serious birth defects — and miscarriages and stillbirths — when it infects pregnant women. So far the most common of these defects appears to be microcephaly, literally “small brain,” which usually leaves surviving children profoundly disabled.
Doctors and epidemiologists in Latin America, where the spread of Zika has been pandemic for the past year or so, first noticed that some abnormal increases in microcephaly cases among newborns followed local outbreaks of Zika. More recently, they have reported isolating the virus from the brains of fetuses with microcephaly who died before birth.
Naturally, the pandemic spread of a virus that appears to threaten children in this way has caused widespread public concern. More than one Latin American government has advised its citizens not to attempt to have babies until the outbreak is over. In the US and other temperate countries, there is fear that Zika could begin spreading via some of the same mosquitoes that currently spread West Nile virus — an encephalitis virus that killed more than 300 in the US in 2012.
The good news is that work on a Zika vaccine is already underway at more than a dozen corporations and government research organizations. A senior official at the US National Institutes of Health (NIH) has stated that one of the NIH candidate Zika vaccines — which incorporates know-how obtained during previous dengue fever virus and West Nile virus vaccine projects — should be ready for clinical trials by early 2017.
The bad news is that even if all goes well with those efforts, an effective, tested vaccine is unlikely to be ready for public use in the next three years. Moreover, researchers seem to have made no progress, so far, in understanding how Zika causes profound abnormalities in brain development or in devising therapies to prevent it. Although the NIH has declared its eagerness to consider grant proposals for Zika research, scientists do not yet have an animal model on which to test their theories, such as mice that can be infected by Zika and later give birth to pups with microcephaly. In fact, developing an animal model is now a key NIH research priority.
“Right now a lot of people want to find out if it’s possible to develop a Zika virus mouse model,” said Jun Huh, a researcher at the University of Massachusetts.
Does Zika infect neural stem cells?
A major line of research on Zika and its mechanisms is informed by prior work on maternal infection with cytomegalovirus (CMV). Currently in the United States, CMV maternal infection is a major, ongoing cause of microcephaly — and also leads to other birth defects including deafness.
CMV is a DNA-based virus and part of the herpesvirus family. Transmissible by close contact including sex, it is widely prevalent in the human population but is typically kept at bay indefinitely by the immune system. However, in a young woman who has only recently been exposed to CMV, or for some reason has a weakened immunity to CMV, the virus may be abundant in her blood — and if the woman is pregnant, her CMV infection may then cross to the bloodstream of the fetus via the placenta.
Research suggests that CMV microcephaly occurs in some of these cases, particularly when infection arises during a critical early period of brain growth — from about 10 to 16 weeks of gestation.
“There’s a defect in neuronal migration” in these cases, said Mark Schleiss, a CMV researcher at the University of Minnesota. “Normally as the brain is developing, different stem cells and precursor cells migrate to different regions and then differentiate into neurons, glial cells, and astrocytes — but that developmental blueprint is disrupted by the virus, and so the brain doesn’t sculpt itself and form normally.”
The result is a brain that is not just too small but also too smooth — lacking the usual deep folds and ridges — and shows enlarged ventricles and abnormal calcium deposits. The microcephaly cases linked to Zika, notes Schleiss, tend to have the same features.
How does CMV bring about this profound derangement of brain development? Schleiss and others suspect that one key mechanism involves neural stem cells. Studies have found that CMV replicates poorly in mature neurons, but relatively efficiently in neural stem cells, and also that CMV infection of the fetal brain tends to be concentrated in areas where neural stem cells are particularly abundant. Thus, the virus could disrupt brain development by directly infecting and killing the very cells that are necessary to populate a child’s brain normally. Cell culture studies suggest that CMV, even when it doesn’t kill infected neural stem cells outright, inhibits their ability to proliferate and mature into working neurons — which would tend to have a similar effect in preventing normal brain growth.
Schleiss noted that CMV infection of the fetal brain also induces a lot of inflammation, which probably contributes as well to the infection’s overall disruption of neural development.
“I suspect that as the story unfolds with Zika virus, we’ll see a similar set of mechanisms,” said Schleiss. “The final pathology is so similar — it’s really very striking.”
Schleiss and his colleagues now hope to develop an animal model of Zika virus, using guinea pigs, which they have previously used to model maternal CMV infection — in part, said Schleiss, because the guinea pig placenta better resembles the human placenta, compared with mice.
The wrong signal?
Probably most research on Zika will investigate mechanisms that involve the virus’s infection and harm of brain cells. But at least one set of researchers, Huh and his colleagues, plan to study the possibility that Zika disrupts fetal brain development also — or even primarily — by sending young brain cells the wrong developmental signals.
Huh has been trying to find the mechanism by which maternal infection causes some children to grow up with a relatively subtle form of abnormal brain development, namely autism or what are now called autism-spectrum disorders.
In a recent study in a mouse model, Huh and his colleagues found evidence that an immune signaling protein, IL-17a, may be the key factor. Abnormally elevated levels of IL-17a, induced via inflammation in the mother and crossing to the brain of the pup in her womb, caused patchy disruptions of cortical development in the fetal brain, along with autism-like behavior after the mice were born. Blocking IL-17 activity prevented both the cortical and behavioral signs.
How IL-17a exerts this deleterious effect on brain development isn’t yet clear. But the researchers did find that it works via IL-17a receptors on cells in the developing brain. That finding raised the possibility that IL-17a and its corresponding brain-cell receptors normally have some role in steering neural developmental processes — apart from their known role in promoting inflammation. In other words, an abnormal surge of IL-17 during brain development, might create an abnormal steering signal and thus derail healthy neural development.
It appears that no one has yet studied IL-17a levels in people infected with Zika virus. But there is indirect evidence to suggest that in some cases those levels will be unusually elevated. Zika infections occasionally lead to cases of the autoimmune disease known as Guillain-Barré syndrome — which is known to feature, and probably is at least partly induced by, high levels of IL-17a (which are associated with other autoimmune disorders as well).
Huh and his colleagues now hope to develop a Zika mouse model to investigate IL-17a’s possible role in causing microcephaly.
“It’s obviously an interesting possibility, especially that this enhanced inflammation in the mother may have some long-term effect in the child,” said Huh.
What if they never find out?
Huh expects that within a year or two, researchers will have working animal models of Zika infection and microcephaly, and will then be able to study those to tease apart the molecular mechanisms.
It is possible, of course, that for the foreseeable future scientists will not understand those mechanisms — particularly if the development of an effective vaccine renders Zika research much less urgent, and so less easily funded.
Schleiss notes that something like that happened with rubella (a.k.a. German Measles) virus, maternal infections of which also can cause microcephaly. “People kind of stopped studying it, because when a successful vaccine came along, they said, ‘Who cares about the mechanism?’” he said. “But as we sit here in 2016 discussing Zika virus — maybe it would have made sense to keep studying rubella to try to understand its mechanism.”
- by Jim Schnabel
CONTENT PROVIDED BY
The Dana Foundation is a private philanthropic organization that supports brain research through grants and educates the public about the successes and potential of brain research.