Is autism a disability, disorder, or difference? And are its associated features considered symptoms, features, or traits? As various communities continue to debate the terminology, autism remains a prevalent condition with wide-ranging phenotypes across people.
Autism is often considered a childhood condition, although many of its symptoms and traits persist lifelong. Some people with autism also have mood and anxiety disorders, seizures, intellectual disabilities, attention deficit hyperactivity disorder (ADHD), or obsessive-compulsive disorder (OCD). However, more than 40% of people with autism have normal or above-average intelligence. With symptoms that range from mildly to severely disabling, autism is considered a spectrum. Autism spectrum disorders (ASD) are diagnosed based on two main criteria: impaired social communication and interaction, and repetitive behaviors or narrow, obsessive interests. For example, some people on the autism spectrum are unable to speak, while others are socially awkward but highly articulate. Many adults with an autism diagnosis think of their autism as a strength — enabling or motivating them to develop deep expertise in an area or a different perspective on the world — rather than a disorder that needs to be cured.
Currently, 1 of every 44* 8-year-olds in the U.S. is estimated to meet the diagnostic criteria for an autism spectrum disorder. The prevalence of ASD has risen dramatically since the 1970s, but it is unclear how much changes to diagnostic criteria and wider recognition of ASD have contributed to the increase in diagnoses.
Four to five times more boys than girls are diagnosed with autism, although it is not clear whether some of that pattern is due to under diagnosis of girls. External factors such as parents having children later in life, fever and infection during pregnancy, and premature births have been linked to an increased risk of autism in children. A huge number of studies have found no connection between childhood vaccination and the increase in autism diagnoses.
Autism is believed to be at least partially driven by genetics, but how do scientists know that for sure? One low-tech approach uses twin studies: If one of a pair of identical twins receives an autism diagnosis, the other twin has greater than a 50% chance of also being diagnosed with ASD. Children who have an older sibling on the spectrum also have a higher likelihood of being diagnosed with autism — nearly 1 in 5 also receives a diagnosis of ASD.
The genetics of autism is very complicated in most cases, involving dozens (or more) of genes, leading to a unique condition in nearly every person. Recently, however, high-throughput genomic analyses have broadened the pool of potential genes, revealed their roles in the body, and suggested possible new therapies.
It appears that many genes, each with a small effect, contribute to the inheritance of most ASDs. But such small effects make these genes hard to identify in genome-wide association studies. Scientists are now looking at the rare variants associated with ASD. These afflict fewer people with ASD, but their effects are larger and easier to detect. Some of these rare mutations are in single genes whose impairment is already known to cause intellectual disability and social dysfunction. These genes include FMR1 (codes for fragile X mental retardation protein, but its non-mutant form is needed for normal cognitive development); PTEN (codes for a tumor suppressor enzyme that regulates cell division, so cells don’t divide or grow too fast); and TSC1 or TSC2 (tuberous sclerosis complex 1 and 2), which also code for proteins that help control cell growth and size. Between 50-60% of people with fragile X syndrome and approximately 40% of people with tuberous sclerosis complex have ASD. Children with a variant of the gene NF-1 develop tumors in childhood (neurofibromatosis) and a 2011 study found that nearly 10% met the criteria for autism.
Intriguingly, these ASD-related genes influence a major signaling pathway for regulating cell metabolism, growth, and proliferation: the mTOR pathway. This suggests a very real potential for treating autism with drugs that target the mTOR pathway. For example, mouse models with mutations in PTEN show traits similar to humans with these gene variants: altered sociability, anxiety, and repetitive behaviors. These behaviors can be relieved or reversed by drugs that inhibit the mTOR pathway. Clinical trials of these drugs (rapamycin and lovastatin) are underway.
Despite this progress, autism genetics is so complicated that it can’t be used to diagnose the condition. And unlike diabetes, kidney disease, or thyroid disease, there are no biochemical or other biomarkers of autism. Currently, autism diagnosis is based on behavioral analysis, but efforts are underway to use more objective criteria such as tracking eye movements and functional neuroimaging, which can even be done in infants.
How early can autism be detected? Parents often notice developmental issues before their child’s first birthday, and autism can be reliably diagnosed based on behavioral characteristics at age 2. Despite these possibilities for early detection, most American children aren’t diagnosed until they’re about 4½ years old. With evidence mounting that interventions are more effective the earlier they begin, researchers are hoping more objective measures will enable earlier diagnoses and interventions.
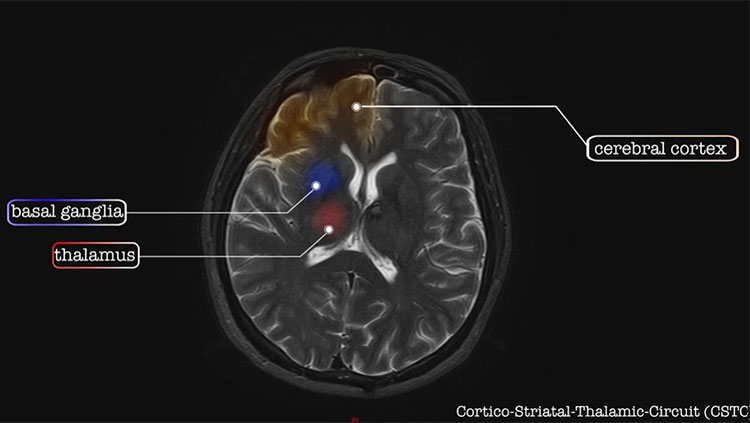
Although the molecular causes and characteristics of autism are unclear, it appears that the condition results from unusual cellular development within the cerebral cortex — a brain region that is crucial to memory, attention, perception, language, and other functions. Both white matter and gray matter of the brain show consistent, but subtle, alterations in people with ASD. Long-term studies also have found that a minority of children on the autism spectrum have abnormally large brain volumes and faster brain growth. Other toddlers with autism have shown unusual development and network inefficiencies at the back of the cerebral cortex. There is evidence that some atypical activity occurs in the cortex of people with ASD from older childhood into adulthood, and information might not be integrated in the usual way across distributed brain networks.
At this point, no medications have been proven to reverse autism. Some people get symptomatic relief from drugs designed for other uses, such as anxiety conditions, and several studies have reported social benefits from treatment with oxytocin — a hormone known to improve social bonding — but the findings have been mixed. For this challenging disorder, behavioral therapies are still the only proven treatments for autism, and early interventions are the most effective.
Adapted from the 8th edition of Brain Facts by Karen Weintraub.
*This statistic was updated March 20, 2023, to reflect the most recent CDC data on ASD prevalence.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Allen, E. G., Freeman, S. B., Druschel, C., Hobbs, C. A., O'Leary, L. A., Romitti, P. A., Royle, M. H., Torfs, C. P., & Sherman, S. L. (2009). Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Human genetics, 125(1), 41–52. https://doi.org/10.1007/s00439-008-0603-8
Barbaresi, W. J., Colligan, R. C., Weaver, A. L., Voigt, R. G., Killian, J. M., & Katusic, S. K. (2013). Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics, 131(4), 637–644. https://doi.org/10.1542/peds.2012-2354
Bishop D. V. (2015). The interface between genetics and psychology: lessons from developmental dyslexia. Proceedings. Biological sciences, 282(1806), 20143139. https://doi.org/10.1098/rspb.2014.3139
Casanova, J. R., Nishimura, M., & Swann, J. W. (2014). The effects of early-life seizures on hippocampal dendrite development and later-life learning and memory. Brain research bulletin, 103, 39–48. https://doi.org/10.1016/j.brainresbull.2013.10.004
Casanova, M. (2015). The Neuropathology of Autism. In: Fatemi, S. (eds) The Molecular Basis of Autism. Contemporary Clinical Neuroscience. Springer, New York, NY. 153-171. https://doi.org/10.1007/978-1-4939-2190-4_8
Christensen, D. L., Baio, J., Braun, K. V., Bilder, D., Charles, J., Constantino, J. N., Daniels, J., Maureen, S. Durkin, M. S., Robert, T., Fitzgerald, R. T., Kurzius-Spencer, M., Lee, L., Pettygrove, S., Robinson, C., Schulz, E., Wells, C., Wingate, M. S., Zahorodny, W., Yeargin-Allsopp, M. (2016). Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ 2016;65(No. SS-3)(No. SS-3):1–23. DOI: http://dx.doi.org/10.15585/mmwr.ss6503a1external icon.
Chronis-Tuscano, A., Molina, B. S., Pelham, W. E., Applegate, B., Dahlke, A., Overmyer, M., & Lahey, B. B. (2010). Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Archives of general psychiatry, 67(10), 1044–1051. https://doi.org/10.1001/archgenpsychiatry.2010.127
Durkin, M. S., Maenner, M. J., Newschaffer, C. J., Lee, L. C., Cunniff, C. M., Daniels, J. L., Kirby, R. S., Leavitt, L., Miller, L., Zahorodny, W., & Schieve, L. A. (2008). Advanced parental age and the risk of autism spectrum disorder. American journal of epidemiology, 168(11), 1268–1276. https://doi.org/10.1093/aje/kwn250
Emerson, R. W., Adams, C., Nishino, T., Hazlett, H. C., Wolff, J. J., Zwaigenbaum, L., Constantino, J. N., Shen, M. D., Swanson, M. R., Elison, J. T., Kandala, S., Estes, A. M., Botteron, K. N., Collins, L., Dager, S. R., Evans, A. C., Gerig, G., Gu, H., McKinstry, R. C., Paterson, S., … Piven, J. (2017). Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Science translational medicine, 9(393), eaag2882. https://doi.org/10.1126/scitranslmed.aag2882
Finn, E. S., Shen, X., Holahan, J. M., Scheinost, D., Lacadie, C., Papademetris, X., Shaywitz, S. E., Shaywitz, B. A., & Constable, R. T. (2014). Disruption of functional networks in dyslexia: a whole-brain, data-driven analysis of connectivity. Biological psychiatry, 76(5), 397–404. https://doi.org/10.1016/j.biopsych.2013.08.031
Fisch, H., Hyun, G., Golden, R., Hensle, T. W., Olsson, C. A., & Liberson, G. L. (2003). The influence of paternal age on down syndrome. The Journal of urology, 169(6), 2275–2278. https://doi.org/10.1097/01.ju.0000067958.36077.d8
Frazier, T. W., Klingemier, E. W., Beukemann, M., Speer, L., Markowitz, L., Parikh, S., Wexberg, S., Giuliano, K., Schulte, E., Delahunty, C., Ahuja, V., Eng, C., Manos, M. J., Hardan, A. Y., Youngstrom, E. A., & Strauss, M. S. (2016). Development of an Objective Autism Risk Index Using Remote Eye Tracking. Journal of the American Academy of Child and Adolescent Psychiatry, 55(4), 301–309. https://doi.org/10.1016/j.jaac.2016.01.011
Geschwind, D. H., & State, M. W. (2015). Gene hunting in autism spectrum disorder: on the path to precision medicine. The Lancet. Neurology, 14(11), 1109–1120. https://doi.org/10.1016/S1474-4422(15)00044-7
Guastella, A. J., & Hickie, I. B. (2016). Oxytocin Treatment, Circuitry, and Autism: A Critical Review of the Literature Placing Oxytocin Into the Autism Context. Biological psychiatry, 79(3), 234–242. https://doi.org/10.1016/j.biopsych.2015.06.028
Hallmayer, J., Cleveland, S., Torres, A., et al. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi:10.1001/archgenpsychiatry.2011.76
Helguera, P., Seiglie, J., Rodriguez, J., Hanna, M., Helguera, G., & Busciglio, J. (2013). Adaptive downregulation of mitochondrial function in down syndrome. Cell metabolism, 17(1), 132–140. https://doi.org/10.1016/j.cmet.2012.12.005
Hornig, M., Bresnahan, M. A., Che, X., Schultz, A. F., Ukaigwe, J. E., Eddy, M. L., Hirtz, D., Gunnes, N., Lie, K. K., Magnus, P., Mjaaland, S., Reichborn-Kjennerud, T., Schjølberg, S., Øyen, A. S., Levin, B., Susser, E. S., Stoltenberg, C., & Lipkin, W. I. (2018). Prenatal fever and autism risk. Molecular psychiatry, 23(3), 759–766. https://doi.org/10.1038/mp.2017.119
Jiang, J., Jing, Y., Cost, G. J., Chiang, J. C., Kolpa, H. J., Cotton, A. M., Carone, D. M., Carone, B. R., Shivak, D. A., Guschin, D. Y., Pearl, J. R., Rebar, E. J., Byron, M., Gregory, P. D., Brown, C. J., Urnov, F. D., Hall, L. L., & Lawrence, J. B. (2013). Translating dosage compensation to trisomy 21. Nature, 500(7462), 296–300. https://doi.org/10.1038/nature12394
Kasparek, T., Theiner, P., & Filova, A. (2015). Neurobiology of ADHD From Childhood to Adulthood: Findings of Imaging Methods. Journal of attention disorders, 19(11), 931–943. https://doi.org/10.1177/1087054713505322
Katsnelson, A., Buzsáki, G., & Swann, J. W. (2014). Catastrophic childhood epilepsy: a recent convergence of basic and clinical neuroscience. Science translational medicine, 6(262), 262ps13.
Kozlowski, A. M., Matson, J. L., Horovitz, M., Worley, J. A., & Neal, D. (2011). Parents' first concerns of their child's development in toddlers with autism spectrum disorders. Developmental neurorehabilitation, 14(2), 72–78. https://doi.org/10.3109/17518423.2010.539193
Lainhart J. E. (2015). Brain imaging research in autism spectrum disorders: in search of neuropathology and health across the lifespan. Current opinion in psychiatry, 28(2), 76–82. https://doi.org/10.1097/YCO.0000000000000130
Lord, C., Risi, S., DiLavore, P. S., Shulman, C., Thurm, A., & Pickles, A. (2006). Autism from 2 to 9 years of age. Archives of general psychiatry, 63(6), 694–701. https://doi.org/10.1001/archpsyc.63.6.694
National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. (2016). Autism Spectrum Disorder (ASD): Data & Statistics. Centers for Disease Control. https://www.cdc.gov/autism/data-research/?CDC_AAref_Val=https://www.cdc.gov/ncbddd/autism/data.html
Nei, M., Ngo, L., Sirven, J. I., & Sperling, M. R. (2014). Ketogenic diet in adolescents and adults with epilepsy. Seizure, 23(6), 439–442. https://doi.org/10.1016/j.seizure.2014.02.015
Norton, E. S., & Wolf, M. (2012). Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annual review of psychology, 63, 427–452. https://doi.org/10.1146/annurev-psych-120710-100431
Ozernov-Palchik, O., & Gaab, N. (2016). Tackling the 'dyslexia paradox': reading brain and behavior for early markers of developmental dyslexia. Wiley interdisciplinary reviews. Cognitive science, 7(2), 156–176. https://doi.org/10.1002/wcs.1383
Ozonoff, S., Young, G. S., Carter, A., Messinger, D., Yirmiya, N., Zwaigenbaum, L., Bryson, S., Carver, L. J., Constantino, J. N., Dobkins, K., Hutman, T., Iverson, J. M., Landa, R., Rogers, S. J., Sigman, M., & Stone, W. L. (2011). Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics, 128(3), e488–e495. https://doi.org/10.1542/peds.2010-2825
Papavassiliou, P., Charalsawadi, C., Rafferty, K., & Jackson-Cook, C. (2015). Mosaicism for trisomy 21: a review. American journal of medical genetics. Part A, 167A(1), 26–39. https://doi.org/10.1002/ajmg.a.36861
Paulesu, E., Danelli, L., & Berlingeri, M. (2014). Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Frontiers in human neuroscience, 8, 830. https://doi.org/10.3389/fnhum.2014.00830
Presson, A. P., Partyka, G., Jensen, K. M., Devine, O. J., Rasmussen, S. A., McCabe, L. L., & McCabe, E. R. (2013). Current estimate of Down Syndrome population prevalence in the United States. The Journal of pediatrics, 163(4), 1163–1168. https://doi.org/10.1016/j.jpeds.2013.06.013
Schendel, D., & Bhasin, T. K. (2008). Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics, 121(6), 1155–1164. https://doi.org/10.1542/peds.2007-1049
Thapar, A., Cooper, M., Eyre, O., & Langley, K. (2013). What have we learnt about the causes of ADHD?. Journal of child psychology and psychiatry, and allied disciplines, 54(1), 3–16. https://doi.org/10.1111/j.1469-7610.2012.02611.x
Tolaymat, A., Nayak, A., Geyer, J. D., Geyer, S. K., & Carney, P. R. (2015). Diagnosis and management of childhood epilepsy. Current problems in pediatric and adolescent health care, 45(1), 3–17. https://doi.org/10.1016/j.cppeds.2014.12.002
Tomasi, D., & Volkow, N. D. (2014). Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cerebral cortex (New York, N.Y. : 1991), 24(4), 935–944. https://doi.org/10.1093/cercor/bhs382
Visser, S. N., Danielson, M. L., Bitsko, R. H., Holbrook, J. R., Kogan, M. D., Ghandour, R. M., Perou, R., & Blumberg, S. J. (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. Journal of the American Academy of Child and Adolescent Psychiatry, 53(1), 34–46.e2. https://doi.org/10.1016/j.jaac.2013.09.001
Willsey, A. J., & State, M. W. (2015). Autism spectrum disorders: from genes to neurobiology. Current opinion in neurobiology, 30, 92–99. https://doi.org/10.1016/j.conb.2014.10.015
Wiseman, F. K., Al-Janabi, T., Hardy, J., Karmiloff-Smith, A., Nizetic, D., Tybulewicz, V. L., Fisher, E. M., & Strydom, A. (2015). A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nature reviews. Neuroscience, 16(9), 564–574. https://doi.org/10.1038/nrn3983
Witton, J., Padmashri, R., Zinyuk, L. E., Popov, V. I., Kraev, I., Line, S. J., Jensen, T. P., Tedoldi, A., Cummings, D. M., Tybulewicz, V. L. J., Fisher, E. M. C., Bannerman, D. M., Randall, A. D., Brown, J. T., Edwards, F. A., Rusakov, D. A., Stewart, M. G., & Jones, M. W. (2015). Hippocampal circuit dysfunction in the Tc1 mouse model of Down syndrome. Nature neuroscience, 18(9), 1291–1298. https://doi.org/10.1038/nn.4072
Young, L. J., & Barrett, C. E. (2015). Neuroscience. Can oxytocin treat autism? Science (New York, N.Y.), 347(6224), 825–826. https://doi.org/10.1126/science.aaa8120
Zuberi, S. M., & Symonds, J. D. (2015). Update on diagnosis and management of childhood epilepsies. Jornal de pediatria, 91(6 Suppl 1), S67–S77. https://doi.org/10.1016/j.jped.2015.07.003
What to Read Next
Also In Childhood Disorders
Trending
Popular articles on BrainFacts.org