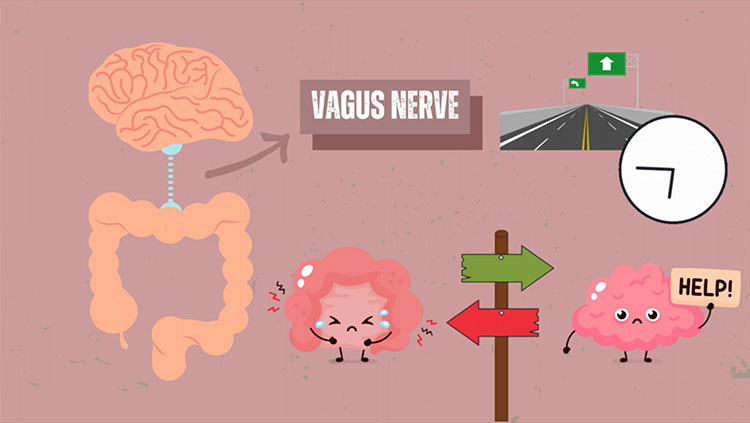
New Study Explores How GLP-1 Analog Administration Affects Running in Mice
- Published17 Dec 2024
- Author Bella Isaacs-Thomas
- Source BrainFacts/SfN
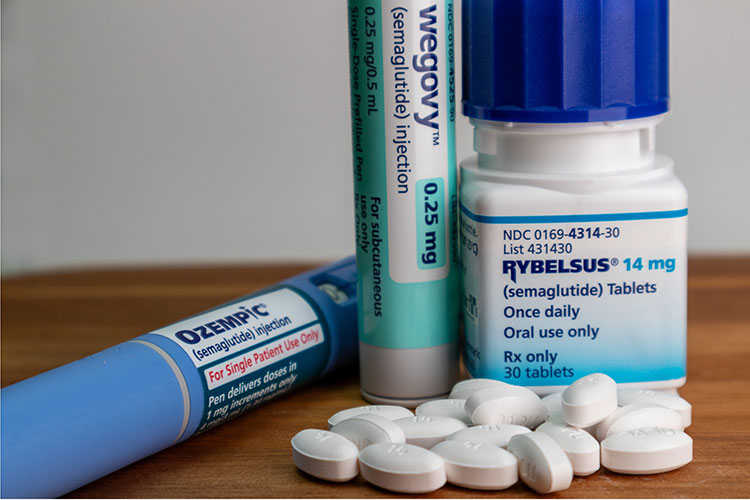
Known mostly for their role as obesity treatments, GLP-1 analogs like Ozempic and Mounjaro are seemingly everywhere. But these drugs have a much longer history. For nearly two decades, they have helped people with type 2 diabetes control their blood glucose. Turns out, they also spur weight loss because they masquerade as a brain-engaging hormone our bodies produce to manage appetite.
In the wake of their growing marketability as a weight-loss tool, researchers are examining other ways GLP-1 drugs may influence our behavior and whether they could treat a broader range of health challenges.
“When we were using these for individuals with type 2 diabetes, we didn't think of them as brain drugs,” said Randy Seeley, a University of Michigan School of Medicine professor of surgery, internal medicine, and nutritional sciences. “You think of them as having effects in the pancreas, but it turns out they're brain drugs.”
A recent study involving semaglutide — a type of GLP-1 analog used in medications like Ozempic and Wegovy — indicated the drug altered the way lab mice approached running on a wheel, a behavior akin to exercise. Albeit, in a manner some may find surprising.
Researchers from Yale measured the effort mice put into the increasingly arduous task of unlocking a running wheel, an indicator of how motivated they were to run, and the total distance they ran on the wheel daily. They observed a drop in that motivation as the mice ran roughly 40% less total distance and made about 25% fewer efforts to unlock the wheel, said Ralph DiLeone, a neuroscientist who presented his team’s preliminary findings at the Society for Neuroscience’s annual meeting in October in Chicago.
When the researchers fed lab mice an obesity-inducing diet and repeated the same experiments, those mice similarly put less effort into unlocking the wheel.
“These agonists are clearly reducing motivation to access the wheels and the total running,” DiLeone said, adding this drop in physical activity could be in line with reductions in food intake and, potentially, reduced alcohol use observed among people taking GLP-1 analogs. But he noted it’s not yet clear why these drugs could have this effect in mice, and he suggested future research could assess whether they could be acting on the brain’s reward and motivation systems or if they’re influencing habitual or compulsive behavior.
There’s no widespread evidence suggesting patients who use GLP-1 analogs exercise less. In fact, people appear to exercise more following chronic treatment with drugs like semaglutide and tirzepatide (Mounjaro), said University of Toronto endocrinologist and professor of medicine Daniel Drucker to BrainFacts via email.
But weight loss does lead to loss of lean mass because as people shed fat, they also lose some of the muscle mass that previously helped support their weight, said Roger Cone, a research professor and director of the University of Michigan’s Life Sciences Institute who wasn’t involved in the study. For people who take GLP-1 analogs over time, there’s still some debate among experts as to whether lean mass loss is just part of generally losing weight, or if the drugs are directly contributing to this loss, he added. A reduction in motivation among GLP-1 drug takers would theoretically be an issue given the importance of exercise in building lean mass and supporting a healthy lifestyle more broadly.
However, in both Cone and Seeley’s view, the daily semaglutide dose DiLeone’s team administered to the mice in their experiment was high enough to cause aversion, or a sick feeling. Cone suggested this malaise could be behind the shift in their running behavior. DiLeone, meanwhile, said the dosing was consistent with those used in other comparable research efforts, and should not have caused significant aversion in mice beyond the first day.
Whether it’s produced by our bodies or introduced by medication, GLP-1 engages with receptors in the hypothalamus and the hindbrain, which are involved with regulating appetite, said Karolina Skibicka, a neuroscientist at Pennsylvania State University. She added they can also target different regions of the brain, too.
“There are also GLP-1 receptors — so there’s the ability to listen to the GLP-1 message — in brain areas outside of those classically metabolic controlling areas,” like the mesolimbic reward system, Skibicka said, which she added could be part of the key to their success as therapeutics. It’s also part of the impetus behind research exploring whether GLP-1 analogs could help treat conditions like addiction.
Cone said the idea these drugs could influence the brain’s reward pathways isn’t a stretch — we need to eat to survive, so we evolved to derive pleasure from the experience as an incentive. But for him, the major question is whether weight loss itself while taking GLP-1 analogs could be the root of various health benefits associated with the drugs, or if they offer more direct benefits beyond appetite reduction.
In coming years, “I think there will be lots of research into these other effects,” Cone said. “And a critical thing will be to understand are these direct effects of GLP-1, or are these effects independent of the weight loss?”
CONTENT PROVIDED BY
BrainFacts/SfN
References
What to Read Next
Also In Body Systems
Trending
Popular articles on BrainFacts.org


.jpg)