Protein Folding: A New Twist on Brain Disease
- Published16 May 2010
- Reviewed16 May 2010
- Author Aalok Mehta
- Source BrainFacts/SfN
Alzheimer’s, Huntington’s, and Parkinson’s are some of the most common brain diseases — each causing a unique form of progressive brain cell death. However, they may not be so different after all. New research suggests these and many other neurological diseases may be versions of the same basic disorder: a breakdown in the body’s ability to fold proteins into their correct shapes. Based on these findings, brain researchers are hoping for a common treatment for these conditions, using new kinds of drugs that prevent misfolding or minimize harm done to the cell.
Many neurodegenerative disorders involve the accumulation of misfolded proteins, although the exact protein involved and the location where it tends to accumulate is specific to each disease.
What if many of the most common brain disorders were all different versions of the same basic disease? At first glance, this seems ridiculous. Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS) have widely varying symptoms, affect different parts of the brain, and respond to different treatments.
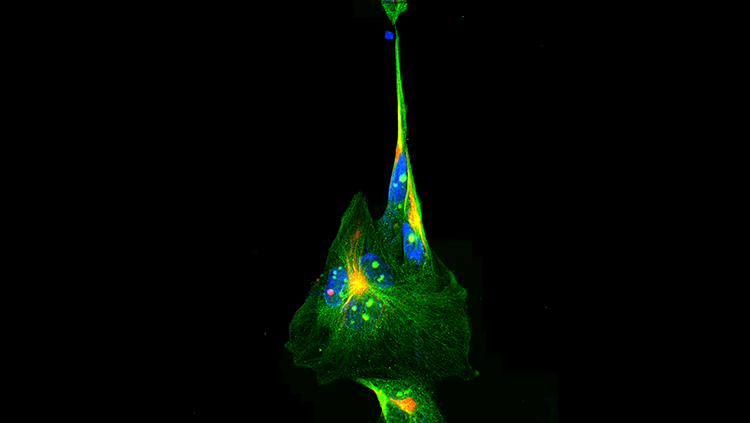
However, a look under the microscope tells a different story. In each of these disorders, brain cells accumulate clusters of misfolded proteins — perhaps the best known are the Lewy bodies found in people with Parkinson’s, the plaques of a protein called amyloid-beta seen in Alzheimer’s patients, or the armies of misfolded proteins called prions in “mad cow” disease. Neuroscientists are finding increasing evidence that these clumps, rather than being a result of disease, may be a root cause.
Research also suggests these clumps develop from the same essential source — a breakdown in the body’s system for ensuring proteins fold into their correct shapes. This theory of “protein misfolding diseases” is leading to:
- Better explanations for how many brain diseases begin, spread, and worsen.
- Potential new, universal methods of treating many disorders by reducing the amount of protein misfolding or by breaking up misfolded proteins.
Proteins are the workhorses of the body. These strings of amino acids provide much of the body’s basic building blocks and internal structure, help essential chemical reactions, allow for communication within and between cells, and provide basic defenses against bacteria and viruses.
Cells make proteins as chains by adding one amino acid at a time based on genetic instructions. Almost immediately, these strings begin to crumple, twist, and rotate into complex, sturdy 3-D shapes, allowing them to fulfill their designated roles.
This process is somewhat delicate, with proteins sometimes getting “stuck” in the wrong form. Cells have therefore developed a sophisticated set of tools for streamlining this folding process, including molecules that assist the folding process and methods for detecting and destroying misfolded proteins.
However, this system sometimes becomes overwhelmed. In the early 1980s, researchers identified misfolded proteins called prions that cause a number of rare brain disorders, such as kuru, bovine spongiform encephalopathy (“mad cow” disease), and Creutzfeldt-Jakob disease. This unprecedented finding led many to wonder, could misshapen proteins be the root cause of other brain diseases?
Brain researchers have now discovered that many brain diseases feature protein folding problems. In Parkinson’s patients, alpha-synuclein forms clumps known as Lewy bodies. Alzheimer’s sees tau tangles form inside brain cells and amyloid plaques accumulate near them. In Huntington’s, the nucleus becomes gummed up with clusters formed by an abnormal version of the huntingtin protein, and in ALS, the proteins superoxide dismutase and TD-43 accumulate in the body and projections of nerve cells.
Studies increasingly suggest these globs of protein, as well as their smaller, more mobile building blocks, are directly responsible for the death of brain cells. In some cases, the affected protein can no longer serve a vital role in the cell or traps other important proteins. Alternatively, the clumps can be directly toxic to cells or interfere with the folding of essential proteins. There also is evidence that the clumps may jumpstart a fatal response by the immune system. Some or all of these probably occur simultaneously, in differing degrees, in different brain diseases.
Other experiments may help explain some mysterious features of neurodegenerative disorders. For instance, with the exception of Huntington’s, each disease can arise spontaneously in people or via inherited genetic mutations, and the severity of symptoms and the speed of the disease can vary tremendously. The overall burden on the folding system may be the culprit; scientists have found that the presence of small, typically harmless mutations in proteins can make clumping worse and appear earlier in animal models. Mutations and misfolding also tend to increase as we age, clearing up why the risk for all of these diseases increases as we get older.
Brain cells also may be particularly susceptible to misfolding diseases. Because neurons are long-lived and do not divide, they may accumulate greater amounts of damaged proteins than other cell types. Also, neurons that die are not usually replaced, magnifying the effect of each lost cell.
How do these misfolded protein diseases spread throughout the brain? Researchers are increasingly looking to prions as a possible model. Prions are like zombies; every time they encounter a correctly folded version of themselves, they cause it to become misfolded, spreading the disease. Like prions, brain researchers are examining whether protein clumps in Alzheimer’s, Huntington’s, and other misfolding diseases spread through the brain in a similar way, explaining why they tend to worsen over time.
Protein folding research may also lead to new treatments for these brain diseases, which currently have no cure. Neuroscientists are already testing drugs that boost the body’s protein-folding system, assist the cell in destroying misfolded proteins, and make it harder for vulnerable proteins to become misfolded in the first place. In the future, scientists may also look at stopping proteins from “infecting” nearby cells.
Although in its early stages, this new work on protein misfolding is already reshaping the way doctors and researchers think about brain disease. In fact, many see it as an essential step toward a way to predict, prevent, and cure neurodegenerative disorders.
CONTENT PROVIDED BY
BrainFacts/SfN
Also In Archives
Trending
Popular articles on BrainFacts.org