Stem Cells: An Overview
- Published24 Mar 2016
- Reviewed24 Mar 2016
- Author Lisa Seachrist Chiu
- Source BrainFacts/SfN
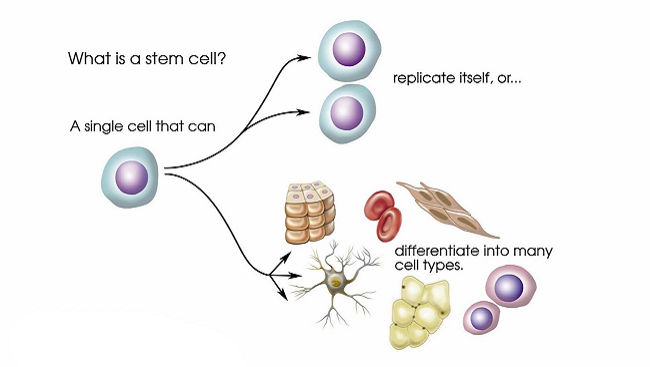
Every person starts life as a single cell with unlimited potential. A stem cell retains that potential and when prodded correctly can become anything from a heart cell to a brain cell. An “induced” stem cell, created from an existing cell, harnesses that flexibility and offers the opportunity to study and perhaps develop new treatments for neurological diseases.
A Cell With Unlimited Potential
After nearly 200 years of peering through ever more powerful microscopes, scientists in the mid-19th century pieced together the implications of what they saw: cells were the building blocks of all life, and the human body developed from a single cell — a fertilized egg. Ever since, scientists have been trying to understand how a single cell could contain and direct such unlimited potential.
In 1868, German biologist Ernst Haeckel coined the term “stem cell” to describe a fertilized egg. Twenty years later, fellow German Theodor Boveri refined that term to describe roundworm embryo cells capable of making copies of themselves (self-renewal) and specializing (differentiating) into any other cell. Boveri’s description comprises the essential characteristics of an embryonic stem (ES) cell. Russian biologist Alexander Maximow theorized in 1909 that a set of “stem cells” in bone marrow could differentiate into red and white blood cells. In effect, he described adult stem cells which renew themselves and serve as a means to repair and maintain tissues like the breast, brain, and bone marrow.
For the next 70 years, scientists made progress in understanding how stem cells worked, but they couldn’t fully study them until 1981 when Gail Martin at the University of California, San Francisco and Martin Evans at the University of Cambridge plucked a cell from a mouse embryo and coaxed it to grow in a petri dish. With a steady supply of mouse ES cells, scientists could study development and disease.
Another breakthrough came in 1998 when James Thomson at the University of Wisconsin-Madison employed a similar technique to grow human ES cells in the lab. The development was heralded by some as the means to one day curing the most intractable degenerative diseases — at the same time it also triggered a robust social debate about the destruction of a human embryo.
Turning Skin Cells Into Neurons
With the ability to become virtually any cell in the body, ES cells captured the popular imagination as a powerful tool to battle neurodegenerative diseases like Parkinson’s disease. At the same time, many researchers cautioned that harnessing the promise of stem cells would take many years. Along with larger ethical debates, that meant the environment surrounding ES cell research remained charged for nearly a decade. During that time scientists also sought other methods to study stem cell development and its potential to treat disease.
In 2006, Shinya Yamanaka at Kyoto University made a discovery that allayed many ethical concerns. His discovery was built on groundbreaking research by John Gurdon at Oxford University in the 1960s that revealed egg cells contained chemical signals capable of reversing the fate of specialized cells to an embryonic-like state. After identifying those chemical signals, Yamanaka inserted four pieces of DNA into a mouse skin cell and triggered the cell to return to a state where it could specialize into any cell type — a state scientists refer to as “pluripotency.” A year later he repeated the feat in a human skin cell and called those cells “induced pluripotent stem cells” or iPSCs for short.
Today, iPSCs can be derived from almost any fully differentiated cell like skin cells or blood cells. They are especially helpful for neuroscientists, providing a powerful tool to explore the biology underlying difficult-to-study disorders like amyotrophic lateral sclerosis (ALS), a progressive degeneration of the neurons controlling muscles and some glands. Researchers can’t pluck out these motor neurons from ALS patients to study; they rely on animal models or testing in cell lines with a number of genetic changes that allow them to survive in the lab. Neither fully reflect the disease in humans. However, deriving iPSCs from a patient’s skin cell and coaxing them to specialize into motor neurons provides researchers with an endless supply of neurons to study — neurons that degenerate in a way that more closely resembles how the disease progresses in people.
New Models for Neurological and Psychiatric Diseases
With a steady supply of highly versatile cells, many people look forward to replacing damaged tissues and organs with lab grown versions one day. While that is likely decades away, iPSCs are already influencing medical research and treatments.
Scientists are using iPSCs to study dozens of diseases including Alzheimer’s, ALS, schizophrenia, and many more. Because neurons derived from iPSCs function very much like regular neurons, forming synapses and becoming electrically active in the laboratory dish, they provide a sophisticated model of disease. With a reliable source of cells mimicking various disorders in the lab, researchers can test drugs to see if they can prevent, stop, or reverse a disease process in iPSC-derived neurons. For example, iPSC-derived neurons from a schizophrenia patient formed fewer synapses than the iPSC-derived neurons from a healthy person. Treating those neurons with the anti-psychotic drug loxapine increased the number of synapses. The technology could provide the biological basis for personalized medicine because while loxapine improved synapse formation in iPSC-derived neurons from a specific patient, it didn’t show the same effect in cells from all schizophrenia patients. However, different drugs worked in other patients.
iPSC technology also presents the opportunity to create cells to replace damaged ones or even to grow new organs. Researchers have had success using iPSCs to treat spinal cord injuries and stroke in mice. They are even working to one day transplant dopamine-producing neurons into the brains of Parkinson’s disease patients. And scientists have gone so far as to grow iPSCs in liquid rather than in a flat dish. When properly prodded, those cells grow into a 3-dimensional, mini version of an organ. Scientists have even grown “mini-brains” where the cells communicate with each other — a tool for improving the fundamental understanding of brain function and how to treat neurological and psychiatric diseases.
While iPSC-based therapies still face a number of hurdles, there is much enthusiasm for this work. Through continued research efforts funded by public and private institutions, scientists can explore the tremendous potential of these cells.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Cashman CR, Lazzerini Ospri L. Induced pluripotent stem cells and motor neuron disease: toward an era of individualized medicine. Journal of Neuroscience. 15;33(20):8587-9 (2013).
Cefalo MG, Carai A, Miele E, Po A, Ferretti E, et al. Human iPSC for therapeutic approaches to the nervous system: present and future applications. Stem Cells International. 2016:4869071 (2016).
Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science.322(5909):1811-5 (2008).
Harding J, Mirochnitchenko O. Preclinical studies for induced pluripotent stem cell-based therapeutics. Journal of Biological Chemistry. 289(8):4585-93 (2014).
Maehle AH. Ambiguous cells: the emergence of the stem cell concept in the nineteenth and twentieth centuries. Notes and Records of The Royal Society Journal of the History of Science. 65(4):359-78 (2011).
Martin GR. Isolation of pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma. Proceedings of the National Academies of Science. 78;12, 7634-7638 (1981).
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126(4):663-76 (2006).
Also In Cells & Circuits
Trending
Popular articles on BrainFacts.org