Epigenetics
- Published1 Nov 2011
- Reviewed1 Nov 2011
- Author Jennifer Carr
- Source BrainFacts/SfN
Is it "nature" or "nurture" that influences behavior and health outcomes? Researchers now know these factors are not independent: experience and environment ("nurture") modify genes ("nature") — a phenomenon known as epigenetics.
Some of these modifications can be passed to the next generation, suggesting it may be possible for our life experiences to affect our children and grandchildren. Recent research finds epigenetics affects normal brain processes — such as development or memory — and abnormal brain processes like depression and disease. More research into this emerging field may lead to novel therapies.
A famine and a fat mouse lead to a breakthrough
"Nature vs. nurture" is often set as a conflict: is it genes or the environment that influences behaviors and health? Decades of research helped scientists learn how the environment has an enormous effect on genes and on subsequent generations — sometimes in ways they never imagined.
From Men to Mice
During the winter of 1944-45, the German army cut off food supplies to the western part of the Netherlands, resulting in a five-month famine. Decades later, researchers noticed something peculiar in the adult children born to mothers who were in the first trimester of pregnancy during the famine — higher rates of obesity and cardiovascular disease compared with the general population. These findings demonstrated that a mother's diet could influence the health of her offspring, but how this information was passed between generations was unknown.
In 2003, scientists studying the agouti mouse — a fat, yellow mouse with an increased tendency for diabetes and cancer — found they could change health outcomes for mice by feeding dietary supplements to their mothers before and during pregnancy. The supplements worked by adding a chemical mark to genes, most notably to those involved in coat color and feeding. The result: obese, yellow mothers bore lean, healthy offspring with brown coats.
From Environment to Genes
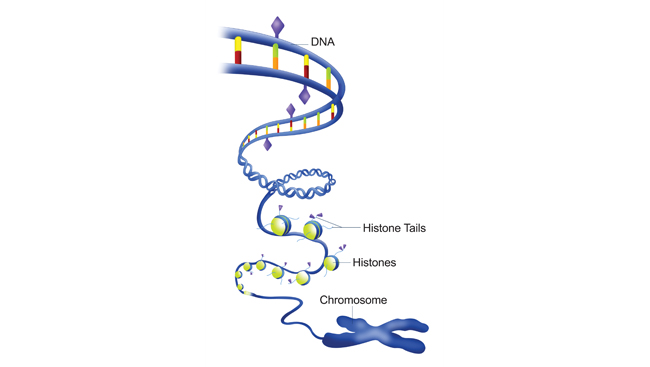
From these and other studies, researchers identified the mechanism by which the environment could modify genetic information, beyond changing the genetic code — the instruction manual for life. One answer seems to be found in how tightly the double helix of DNA is wound.
When not in use, DNA is tightly wound on protein spools inside the cell. Cells are constantly adding or removing chemical marks — usually methyl groups to the DNA or acetyl groups to the spools. Some marks allow the tightly coiled DNA strands to unwind, exposing their genetic sequence. Others encourage the DNA to coil tighter, ensuring the gene remains off-limits.
As early as the 1970s, cancer researchers knew this process had a role in cell growth, but the effect it might have on the brain and behavior was unclear.
Animal research suggests lifetime experience affects brain and behavior
Experiences and the environment change the chemical marks (epigenetic tags) on DNA, and this dictates whether the DNA is unwound and available or tightly wound and off-limits. Why is this important? Cells can turn on genes in loosely coiled DNA, but it is difficult to turn on a gene that is hiding in a tight spool.
What does this mean for the brain and behavior? Cells might have a harder or easier time turning on genes involved in feeding or emotion, for example, because of epigenetic tags.
Experience Shapes Behavior and Memory
Researchers found early life experience influenced stress and fear behaviors in rats through this process. Several studies tracked the interactions between mother rats and their newborn pups, drawing distinctions between those that showed excessive and deficient maternal care (amounts of time grooming and nursing pups). Rats raised by excessive care mothers displayed less fear and stress behaviors as adults.
Importantly, the researchers found differences in the epigenetic tags on a gene related to stress in the different groups of pups as early as the first week of life. These differences remained into adulthood. However, blocking the addition of epigenetic tags reversed the effect of maternal care.
Epigenetic changes in brain cells also affect the ability to form memories, according to other animal research. One rat study explored brain cells in the hippocampus, a region important in memory. It revealed that undertaking a memory task turned on a gene for an enzyme involved in marking DNA. Blocking the enzyme hindered the ability to form the memory.
Researchers also found that an "enriched environment," complete with toys, exercise, and extra attention, improved memory in mice genetically altered to have memory deficits. What's more, the offspring of these mice benefited from the enriched environment, though they had never experienced it themselves.
These early findings point to a role for epigenetics in brain processes and behavior. Only with ongoing research will neuroscientists understand the full extent of epigenetics' reach.
Exploring epigenetics in human behavior and disease
Emerging studies in people suggest epigenetics may affect human behavior and be a factor in neurological and psychiatric disease. For instance, researchers found the same gene affected by parental care in rats also was affected by adverse early life events in humans. These findings suggest early life experiences lead to epigenetic changes in stress-related genes in the human brain. In this way, epigenetics may shape human behaviors, translating life events to a heritable impact on genes.
Epigenetic Key to a Human Disease
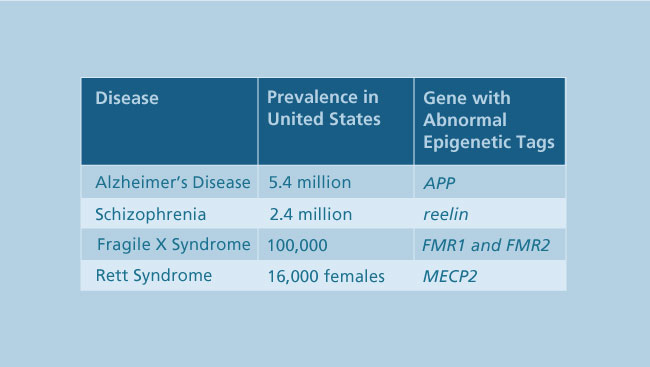
In addition to shaping behavior, epigenetics also may be involved in brain diseases and disorders. One example is Rett syndrome, a genetic disorder that almost exclusively affects young girls and currently has no cure. In its early stages, Rett syndrome causes autistic-like behaviors; in later stages, girls with Rett syndrome lose the ability to speak or control movement.
Research showed Rett syndrome was caused by a mutation in the MECP2 gene. The MeCP2 protein binds to and turns off genes with epigenetic tags. Without proper MeCP2 function, some genes get out of sync. By identifying and manipulating other proteins that perform functions similar to MeCP2, researchers hope to one day improve treatment options for Rett syndrome.
Ongoing research is finding epigenetics may be a key factor in other brain diseases, like schizophrenia, autism, and Alzheimer's disease, indicating the importance of identifying epigenetic patterns throughout the genome and how they are changed by disease. Unlike genetic mutations, epigenetic marks can be reversed. In fact, the U.S. Food and Drug Administration has already approved several drugs that work to improve health outcomes by modifying these marks.
Like much of what we now know about epigenetics, many of these drugs were originally identified by cancer researchers. With these drugs as a foundation, brain scientists are now working to develop safer, more effective drugs to improve cognitive function and behavior in people — highlighting the importance of collaboration across scientific institutes and disciplines and the powerful potential to apply basic and applied research well beyond its original intent.
CONTENT PROVIDED BY
BrainFacts/SfN
Also In Archives
Trending
Popular articles on BrainFacts.org