Storing Memories in Your Synapses
- Reviewed11 Oct 2018
- Author Deborah Halber
- Source BrainFacts/SfN
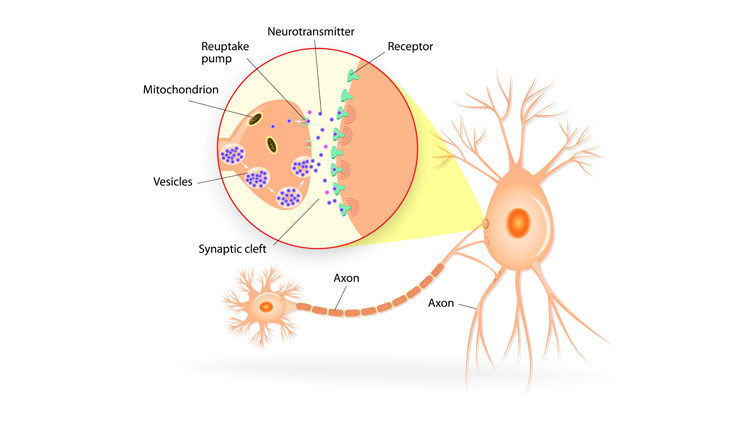
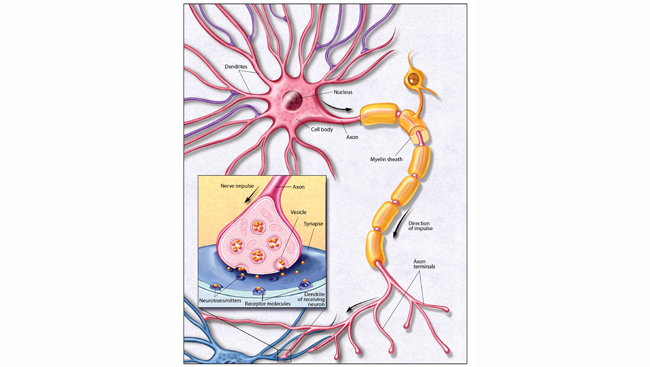
Your ability to recall the color of your childhood home depends on long-lasting changes in your brain. Forming a new memory requires rerouting nerve fibers and altering synapses, the tiny gaps across which neurons relay chemical messages. The ability of synapses to change, or remodel, themselves is called synaptic plasticity. Encoding a new long-term memory involves persistent changes in the number and shape of synapses, as well as the number of chemical messages sent and molecular docking stations, or receptors, available to receive the messages.
Neurons communicate in a stepwise sequence of events. First, an electrical signal in a neuron triggers the release of chemical messengers — called neurotransmitters — from its axon terminals. Those neurotransmitters from the “sending,” or presynaptic, neuron diffuse across the synaptic gap to the “receiving,” or postsynaptic, neuron. There, they interact with receptors embedded in the membrane of the postsynaptic neuron. The interaction is a bit like a lock and key — the right neurotransmitter (key) can unlock and activate the receptor (lock). Upon binding neurotransmitters, receptors unleash a cascade of molecular events that convert the message back into an electrical signal. The receptors then release the neurotransmitters, which are recycled back into the presynaptic terminal or broken down enzymatically, allowing postsynaptic receptors to receive new signals from the presynaptic neuron.
Two opposing but equal processes are key for synaptic plasticity: long-term potentiation (LTP) and long-term depression (LTD). LTP is a long-lasting increase in synaptic strength, which occurs in many brain regions but especially in the hippocampus.
LTD, conversely, decreases a synapse’s effectiveness. Without LTD, you wouldn’t be able to learn anything new or form new memories because synapses would reach a maximum level of strength, after which they’d no longer be plastic. Experience physically changes our brains through both LTP and LTD, shown in numerous animal and human studies to be essential for long-term memory consolidation.
LTP occurs throughout the brain, but it has been studied most extensively in the hippocampus, the brain region associated with encoding new memories. How this is accomplished depends upon the type of neuron. In general, LTP involves an increase in the number of glutamate receptors on the postsynaptic neuron. Glutamate is the most prevalent neurotransmitter in the mammalian nervous system, and it binds to several different kinds of receptors. The NMDA (N-methyl-d-aspartate) and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) classes of glutamate receptors are ion channels. Upon binding glutamate, they permit calcium and sodium ions, respectively, to flow into the cell. Increasing the number of receptors on the postsynaptic cell strengthens a synapse by allowing more electrically conductive ions to enter.
Calcium ions also function as second messengers — signaling molecules that set off a chain of molecular events within cells. LTP boosts the concentration of calcium ions inside a postsynaptic cell, while LTD increases it to a lesser degree. The differing concentrations of calcium activate different enzymes: kinase proteins in the case of LTP, or phosphatases for LTD. These enzymes modify the synapse, making it more or less efficient at relaying nerve impulses.
LTP involves a series of molecular events stabilizing the synaptic changes: The increase in calcium ions within the postsynaptic cell activates cyclic adenosine monophosphate (cAMP) molecules. This, in turn, activates several kinds of enzymes, some of which increase the number of synaptic receptors, making the synapse more sensitive to neurotransmitters. In addition, continued stimulation through repetitive experience activates a molecule called cAMP-response binding element, or CREB. CREB acts in the nucleus of a neuron to switch on a series of genes, many of which direct protein synthesis. Among the many proteins produced are neurotrophins, which stimulate the growth of the synapse and structural elements, stabilizing increased sensitivity to neurotransmitters. This molecular cascade is essential for memories to become long-lasting. The prevailing view is that declarative memories are encoded in the hippocampus, then transferred to the frontal lobes for long-term storage and consolidation. Research suggests that, over time, the hippocampus becomes less important for retrieving older memories as the frontal cortex assumes that task.
As researchers gain new insights into the molecular mechanisms underlying memory, pharmaceutical and technological advances may enable artificial manipulation of synaptic plasticity. New treatments could be developed for synapse-related neurological disorders — such as eradication of harmful memories tied to post-traumatic stress disorder (PTSD) — or for boosting our ability to learn and remember.
This article was adapted from the 8th edition of Brain Facts by Deborah Halber.
CONTENT PROVIDED BY
BrainFacts/SfN